解密“无形之墙”——表面科学 Decoding the "Invisible Wall" — The Science of Surfaces 1.1 界面张力的本质 (γ) 1.1 The Nature of Interfacial Tension (γ) 要理解乳液,首先必须理解阻止它们自然形成的力:界面张力。从根本上说,界面张力是在两种不相溶液体(如油和水)的界面上增加单位面积所需的能量 。想象一下一杯水。在水体内部,每个水分子都被其邻居从四面八方平等地拉扯,这些是内聚力(cohesive forces)。然而,在水面上的分子则不同。它们下方和侧面有水分子拉扯,但上方只有空气。这种不平衡的拉力将表面分子向内拉,形成一个紧密、收缩的表面,就像一层有弹性的薄膜。这就是表面张力(surface tension)。 To understand emulsions, one must first grasp the force that prevents them from forming naturally: interfacial tension. Fundamentally, interfacial tension is the energy required to increase the area of the interface between two immiscible liquids, like oil and water. Imagine a glass of water. Deep within the bulk of the liquid, each water molecule is pulled equally in all directions by its neighbors—these are cohesive forces. The molecules at the surface, however, are different. They are pulled by water molecules below and to their sides, but only by air above. This imbalanced pull draws the surface molecules inward, creating a tight, contractile surface that acts like an elastic membrane. This is surface tension. 当油与水相遇时,同样的情况发生在它们的边界或“界面”上。水分子之间的内聚力远强于水分子和油分子之间的粘附力(adhesive forces) 。因此,两种液体都强烈地倾向于最小化它们之间的接触。这种最小化接触的驱动力表现为界面张力,通常用希腊字母gamma(γ)表示。对于纯油和水,这个值通常在30-50 mN/m的范围内,这是一个相当大的能量壁垒,解释了为什么它们会如此迅速地分离 。一个很好的类比是想象两个紧密团结的人群,他们都更喜欢与自己人互动,并抵制与对方团体混合。他们相遇的边界会很紧张,并且会尽可能地小。 When oil meets water, this same scenario plays out at their boundary, or "interface." The cohesive forces among water molecules are much stronger than the adhesive forces between water and oil molecules. As a result, both liquids strongly prefer to minimize their contact with each other. This drive to minimize contact manifests as interfacial tension, often denoted by the Greek letter gamma (γ). For pure oil and water, this value is typically in the range of 30-50 mN/m, a significant energy barrier that explains why they separate so readily. A good analogy is to imagine two separate, tight-knit crowds of people who each prefer sticking to their own kind and resist mixing with the other group. The boundary where they meet is tense and kept as small as possible. 1.2 和平缔造者:乳化剂与表面活性剂简介 1.2 The Peacemakers: An Introduction to Emulsifiers and Surfactants 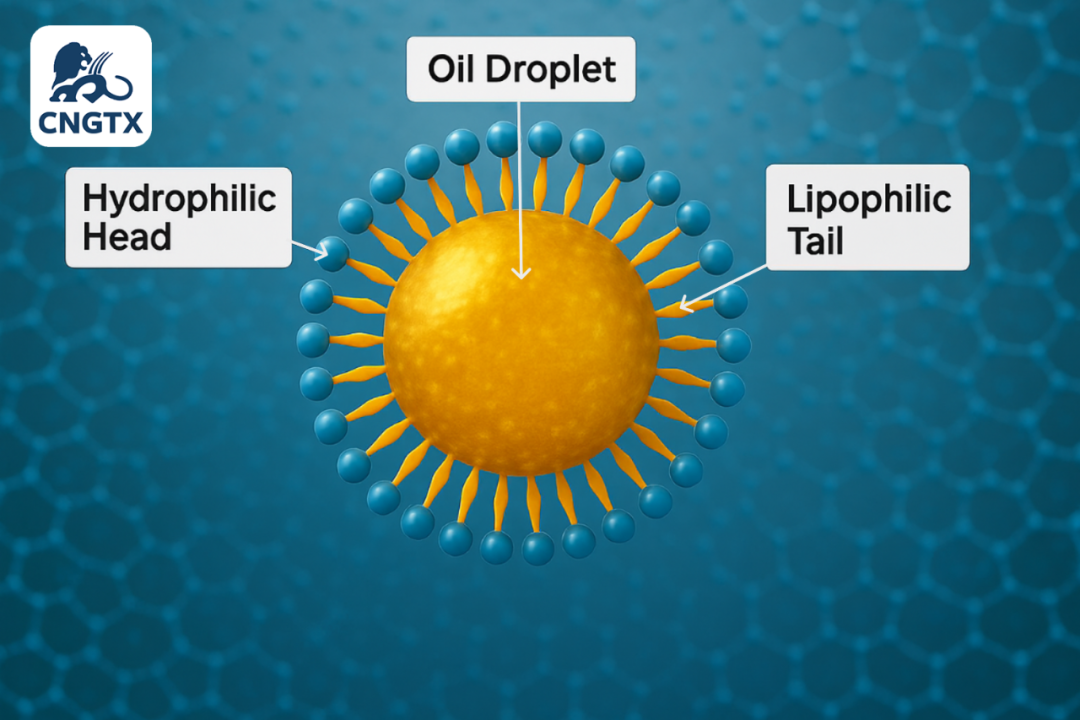
如果界面张力是阻止油和水混合的墙,那么乳化剂就是用来拆掉这堵墙的工具。乳化剂,在化学上更广泛地称为表面活性剂(surfactants,是“surface active agents”的缩写),是能够显著降低界面张力的非凡分子 。它们的秘密在于其独特的两亲性(amphiphilic)或双极性结构。每个表面活性剂分子都有两端:一个亲水(hydrophilic)的“头”,它喜欢水;以及一个亲油(lipophilic)或疏水(hydrophobic)的“尾”,它喜欢油并排斥水。 If interfacial tension is the wall preventing oil and water from mixing, then emulsifiers are the tools used to tear that wall down. Emulsifiers, known more broadly in chemistry as surfactants(a portmanteau of "surface active agents"), are remarkable molecules that can dramatically lower interfacial tension. Their secret lies in their unique amphiphilic, or bipolar, structure. Every surfactant molecule has two ends: a hydrophilic("water-loving") head and a lipophilic or hydrophobic ("oil-loving" or "water-fearing") tail. 当被引入油水混合物中时,这些分子会做一些非常聪明的事情。它们会自然地迁移到油水界面。在那里,它们会自我排列,将亲水的头伸入水相,将亲油的尾伸入油相。这种排列方式有效地在油和水之间形成了一个中介层。它们通过用低能量的乳化剂所实现的相互作用,取代油水之间高能量的直接接触,打破了界面上分子紧密排列的结构,从而将界面张力从30-50 mN/m显著降低至1 mN/m甚至更低。通过这样做,它们围绕着分散的液滴(例如,水中的油滴)形成了一层保护膜或“襁褓”,阻止它们重新接触和合并。 When introduced into an oil-and-water mixture, these molecules do something very clever. They naturally migrate to the oil-water interface. There, they orient themselves with their hydrophilic heads in the water phase and their lipophilic tails in the oil phase. This arrangement effectively forms an intermediary layer between the oil and water. They disrupt the tight molecular packing at the interface by replacing the high-energy direct contact between oil and water with lower-energy emulsifier-mediated interactions, drastically reducing the interfacial tension from 30-50 mN/m to 1 mN/m or even lower. In doing so, they form a protective film or "swaddle" around the dispersed droplets (e.g., oil droplets in water), preventing them from touching and merging again. 1.3 完美乳液的两大支柱:易形成性与稳定性 1.3 The Two Pillars of a Perfect Emulsion: Ease vs. Stability 创造一个成功的乳液不仅仅是降低界面张力。这是一个两阶段的战略胜利,需要解决两个截然不同的挑战:形成的难易程度和长期的稳定性。这是一个至关重要的区别,是理解和设计从蛋黄酱到钻井液等各种乳液的关键 。 Creating a successfulemulsionis about more than just loweringinterfacial tension. It is a two-stage strategic victory that requires solving two distinct challenges: theease of its formationand itslong-term stability. This is a critical distinction and the master key to understanding and designing emulsions in everything from mayonnaise to drilling fluids. 支柱一:乳化的易形成性——降低能量壁垒 Pillar 1: Ease of Emulsification - Lowering the Energy Barrier 乳化的物理过程是将一种液体破碎成微小液滴,并将其分散到另一种液体中,这极大地增加了两种不相溶液体之间的总界面面积。这个过程需要能量输入——无论是通过摇晃、搅拌还是高科技均质机。所需能量(W)与界面张力(γ)和增加的界面面积(ΔA)成正比,其关系可以用一个简单的公式表示:W = γ · ΔA。当γ很高时,需要巨大的能量输入才能创造出新的界面。即使你成功了,系统也处于高能、极不稳定的状态,会迅速恢复到分离状态。通过引入乳化剂来降低γ,形成相同数量的微小液滴所需的能量也大大减少。低界面张力使液滴更容易在剪切力下变形和破碎,从而使乳化过程本身变得更加高效和节能。 The physical act of emulsification involves breaking one liquid into tiny droplets and dispersing it throughout another, which vastly increases the total interfacial area between the two immiscible liquids. This process requires an input of energy—be it from shaking, whisking, or a high-tech homogenizer. The amount of energy required (W) is directly proportional to the interfacial tension (γ) and the increase in interfacial area (ΔA), a relationship captured by the simple formula: W = γ · ΔA. When γ is high, a tremendous amount of energy input is needed to create a new interface. Even if you succeed, the system is in a high-energy, highly unstable state and will rapidly revert to separation. By introducing an emulsifier to lower γ, the energy required to form the same number of tiny droplets is dramatically reduced. Low interfacial tension makes the droplets easier to deform and shatter under shear forces, making the emulsification process itself far more efficient and less energy-intensive. 支柱二:长期稳定性——构建动力学堡垒 Pillar 2: Long-Term Stability - Building a Kinetic Fortress 然而,降低界面张力只是“入场券”。除了极少数例外,如微乳液(microemulsions),它们具有超低的界面张力并且是热力学稳定的,所有常见的乳液(宏乳液)在热力学上都是不稳定的。这意味着它们始终存在一种自然的、不可避免的趋势,即通过液滴合并(聚并)来减少总界面面积,从而降低系统的总能量。一个乳液之所以能保持稳定,不是因为它达到了能量最低的状态,而是因为乳化剂在液滴周围建立了一个强大的防御系统——一个动力学屏障(kinetic barrier)。这个屏障并不能消除分离的趋势,但它能极大地减缓分离过程,使其在实际应用中(数天、数月甚至数年)可以忽略不计。这种稳定性主要来自三个关键机制: Lowering the interfacial tension is merely the "entry ticket," however. With rare exceptions like microemulsions, which have ultra-low interfacial tension and are thermodynamically stable, all common emulsions (macroemulsions) are thermodynamically unstable. This means they always possess a natural, inescapable tendency to reduce the total interfacial area by allowing droplets to merge (coalesce), thereby lowering the system's total energy. An emulsion remains stable not because it has reached its lowest energy state, but because the emulsifier builds a powerful defense system around the droplets—a kinetic barrier. This barrier does not eliminate the drive to separate, but it slows the process down so dramatically that it becomes negligible on a practical timescale (days, months, or years). This stability comes primarily from three key mechanisms: 空间位阻稳定(Steric Stabilization):大型、笨重的乳化剂分子,如蛋白质和聚合物,在液滴表面形成一层厚实、有弹性的物理屏障。当两个液滴试图靠近时,这些保护层会相互挤压,产生排斥力,像保险杠一样阻止它们接触和合并。这层膜的机械强度至关重要 。 Steric Stabilization: Large, bulky emulsifier molecules like proteins and polymers form a thick, elastic physical barrier on the droplet's surface. When two droplets try to approach, these protective layers press against each other, creating a repulsive force that prevents them from making contact and merging, much like bumpers on a car. The mechanical strength of this film is crucial. 静电稳定(Electrostatic Stabilization):离子型表面活性剂会赋予液滴表面电荷(例如,全部带负电)。由于同种电荷相互排斥,液滴之间会产生一种静电斥力,使它们保持距离。Zeta电位是衡量这种排斥力强度的指标,Zeta电位越高,乳液越稳定 。 Electrostatic Stabilization: Ionic surfactants impart an electrical charge (e.g., all negative) to the surface of the droplets. Since like charges repel, the droplets push each other away, creating an electrostatic repulsion that keeps them at a distance. The Zeta potential is a measure of the strength of this repulsion; a higher Zeta potential indicates a more stable emulsion. 吉布斯-马兰戈尼效应(Gibbs-Marangoni Effect):这是一个巧妙的“自愈”机制。如果两个液滴相互靠近,它们之间的液膜变薄,这会改变该区域乳化剂分子的浓度,从而产生界面张力梯度。液体会自发地从低界面张力区域流向高界面张力区域,以平衡这种差异。这种流动会拖拽周围的液体和乳化剂分子进入变薄的区域,从而修复薄弱点,将液滴推开 。一个宏观的例子是“挂杯”现象,其中酒精的蒸发在酒杯壁上产生表面张力梯度,导致液体向上爬升。 Gibbs-Marangoni Effect: This is a clever "self-healing" mechanism. If two droplets approach and the liquid film between them thins, this changes the concentration of emulsifier molecules in that area, creating a gradient in interfacial tension. Liquid will spontaneously flow from regions of low interfacial tension to regions of high interfacial tension to equalize this difference. This flow drags surrounding liquid and emulsifier molecules into the thinned area, effectively healing the weak spot and pushing the droplets apart. A macroscopic example of this is the "tears of wine" phenomenon, where the evaporation of alcohol creates a surface tension gradient on the wall of a wine glass, causing the liquid to climb upwards. 最终,一个乳液的成功取决于这两个支柱的协同作用。降低界面张力使得乳化在能量上成为可能,而一个强大的动力学屏障则确保了乳液在所需的时间内保持其功能和美观。一个界面张力极低但界面膜脆弱的系统可能会在初期形成微小的液滴,但很快就会失效。相反,一个界面张力稍高但具有坚固界面膜的系统可能非常稳定。 Ultimately, the success of an emulsion depends on the synergy of these two pillars. Lowering interfacial tension makes emulsification energetically possible, while a robust kinetic barrier ensures the emulsion remains functional and aesthetically pleasing for its required lifetime. A system with extremely low interfacial tension but a flimsy interfacial film might form tiny droplets initially but will fail quickly. Conversely, a system with a slightly higher interfacial tension but a very robust interfacial film can be exceptionally stable. 现在我们已经解密了界面张力的“无形之墙”,并认识了被称为乳化剂的分子“和平缔造者”,我们有了科学基础来观察这些原理的实际应用。在下一集中,我们将从理论走向具体——而且是美味的——我们将走进厨房,探索掌握乳化技术如何创造出我们最喜欢的一些食物。 Now that we've decoded the "invisible wall" of interfacial tension and met the molecular "peacemakers" known as emulsifiers, we have the scientific foundation to see these principles in action. In our next installment, we'll move from the theoretical to the tangible—and delicious—as we step into the kitchen to explore how mastering emulsions creates some of our favorite foods. | 
