点击蓝字 关注我们

欢迎各位专家学者在公众号平台报道最新研究工作,荐稿请联系小编Robert(微信ID:BrainX007); 或将稿件发送至lgl010@vip.163.com。
英文标题:Highly Stretchable, Adhesive, and Conductive PEDOT Nanocomposite Hydrogels for High-Performance Flexible Bioelectronics
成果简介
近年来,智能医疗的快速发展推动了生物电子学的迅速发展。在各种生物电子应用中,记录心电(ECG)、肌电(EMG)、脑电(EEG)等电生理信号,在推进神经动力学和各种生理过程的理解方面发挥着关键作用。然而,由硅或金属基材料制成的传统传感器具有干燥、刚性、生物相容性差等固有特性,严重阻碍了它们与软组织的集成,并限制了其对下一代柔性生物电子的适用性。导电水凝胶因其类似皮肤的特性和生物相容性,已成为下一代生物电子器件的理想候选材料。然而,它们的实际应用仍然受到其较低电导率和传统导电填料的疏水性等限制,这些因素阻碍了生物电子应用中多种核心功能的集成。
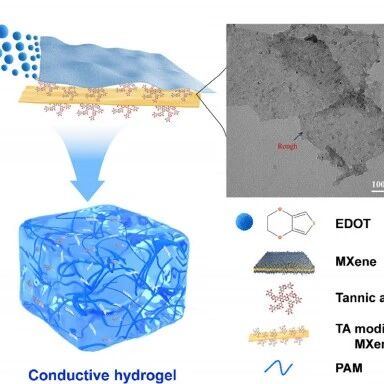
本研究提出了一种高效策略,通过使用单宁酸修饰MXene,原位引发3,4-乙烯二氧噻吩(PEDOT)聚合,制备出导电且可水溶性多功能纳米填料(MX-T-P纳米片)。单宁酸修饰不仅引入大量邻苯二酚基团,显著提升其亲水性与分散性,同时还赋予 PEDOT 复合纳米片固有导电性(385 S·m-1)。作为多功能填料,该纳米片的掺入使得HMXTP水凝胶同时具备优异的拉伸性能(>800%)、强粘附性(≈22kPa)以及高电导率(≈125 S·m-1),综合性能超越传统 PEDOT:PSS 基导电水凝胶,可在体内外实现可靠、高分辨的电生理信号采集。该方法为柔性生物电子材料的开发提供了有效路径,并拓展了水凝胶在生物电子学的应用潜力。
研究亮点
填料设计:利用单宁酸(TA)修饰的MXene,引发EDOT单体在其表面原位聚合,形成结构均匀的PEDOT复合纳米片。增强MXene的亲水性和分散性,促进PEDOT的有序排列,显著提升导电性。
多性能提升:突破了传统填料只能单一提升导电性或机械性能的局限,该填料能够对水凝胶的导电性能、机械性能以及粘附性进行多方位提升。
图文解析

Fig.1. Design strategy, properties, and bioelectronic applications of PEDOT nanocomposite conductive hydrogels. (a)Phase-separated morphology of Mxene-PEDOT. Scale bar for PEDOT: 100 nm, for MXene-PEDOT: 200 nm. (b)In situ polymerization of EDOT on MX-T template followed by incorporation into PAM hydrogel matrix. Scale bar for MX-T-P: 100 nm.(c)Schematic illustration of the intermolecular interactions between MX-T-P nanocomposites and PAM chains. (d,e)The PEDOT nanocomposite conductive hydrogel exhibits self-adhesion and stretchability. (f,g)Application of the PEDOT nanocomposite conductive hydrogel in electrophysiological signal acquisition.

Fig.2. Characterization of PEDOT nanocomposites. (a)TEM image of MXene nanosheets dispersed in aqueous solution. (b)AFM image and height profile of MXene nanosheets. (c)XPS spectra of C 1s for MXene and MXene. (d)TEM image of PEDOT synthesized via conventional oxidative polymerization. (e)TEM image of MXene-PEDOT. (f)TEM image of MX-T-P nanocomposites. (g)AFM image and height profile MX-T-P nanocomposites. (h)Zeta potential of aqueous solutions of PEDOT, MXene, MX-T, and MX-T-P. (i)Dispersion of PEDOT, MXene, and MX-T-P nanomaterials in water. (j)XPS spectra of MX-T-P and MX-T. (k)High-resolution C1s XPS spectra of MX-T-P. (l)The electrical conductivity of PEDOT and MX-T-P nanosheets.

Fig.3. Mechanical and adhesive properties of HMXTP hydrogels. (a)The micromorphology of PAM and HMXTP hydrogels. (b) Photographs of HMXTP hydrogel before and after stretching at a large strain. (c)and (d)Mechanical properties of PAM and HMXTP hydrogels with different nanosheet contents. (e)Comparative of different hydrogel-skin adhesion interfaces. (f)The summary of the shear strength of PAM and HMXTP hydrogels. (g)Macroscopic images of HMXTP hydrogel adhesion on different material surfaces. (h)Adhesion mechanism of HMXTP hydrogel. (i)The shear strength of HMXTP hydrogels adheres to different engineering solids and biological tissues.

Fig.4. Conductivity and bioelectrical signal recording performance of the HMXTP hydrogels. (a)Schematic illustration of the conductivity mechanism of HMXTP hydrogel. (b)The conductivity of different hydrogels. (c)Comparison of real-time ECG signals between HMXTP bioelectrodes and commercial electrodes at rest and after exercise. (d)Comparison of SNR between HMXTP electrode and commercial electrode in resting and post-exercise ECG signal acquisition. (e)EMG signals measured by HMXTP bioelectrodes at different grip forces (30, 40, 50 lb). (f)Comparison of EMG signal performance between the HMXTP bioelectrode and the commercial electrode under 24 kg grip force. (g)Photos showing the influence of the commercial electrode and HMXTP bioelectrode on the skin after wearing for 6 h.

Fig.5. The in vivo bioelectrical signal detection of HMXTP conductive hydrogels. (a)and (b)Schematic illustration and photograph of an ECG recording. (c)ECG signals acquired by HMXTP bioelectrodes. (d)Schematic illustration of EMG recording. (e)EMG signals acquired by HMXTP bioelectrodes. (f)Schematic illustration of EEG recording. (g)EEG signals collected by HMXTP bioelectrodes when the rat was walking or chewing.

Fig.6. A comparative analysis of conductivity, maximum tensile strain, and adhesive strength between this work and recently reported conductive hydrogels.
研究结论
本研究提出了一种通过采用TA改性的MXene作为功能模板来引发EDOT的原位聚合,以制备PEDOT复合纳米片。TA改性增加了MXene表面的表面活性位点密度,从而增强了与EDOT的界面相互作用。获得的MX-T-P纳米片表现出优异的水分散性和电导率,实现了高效的电荷传输。且受益于其优异的分散性,MX-T-P纳米片可以均匀地掺入PAM水凝胶基质中,形成HMXTP水凝胶,使其水凝胶显示出优异的机械性能和粘附性能。MX-T-P纳米片作为多功能填料,使HMXTP水凝胶能够同时实现高拉伸性、强粘附性和高电导率这些协同特性,且能够稳定和高保真地采集ECG和EMG电生理信号,突出了该水凝胶在可穿戴生物电子应用方面的潜力。这项研究不仅解决了高性能导电水凝胶开发中的痛点,还为推进基于水凝胶的下一代生物电子器件和应用提供了一种有前景的方法。
免责声明:原创仅代表原创编译,水平有限,仅供学术交流,如有侵权,请联系删除,文献解读如有疏漏之处,我们深表歉意。


公众号丨智能传感与脑机接口